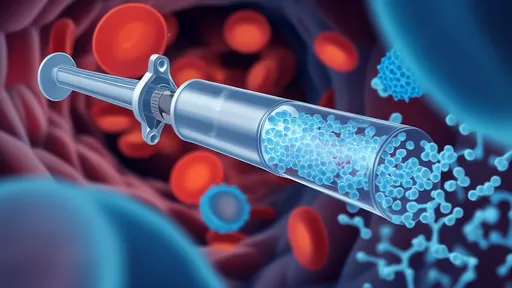
In a groundbreaking advancement that could revolutionize cancer treatment, researchers have unveiled a bioinspired hydrogel system capable of trapping circulating tumor cells (CTCs) like a biological "freeze gun." This innovative approach, modeled after natural mechanisms, offers new hope for preventing metastatic spread—the primary cause of cancer-related deaths worldwide.
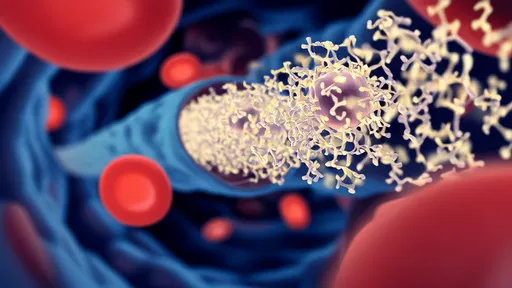
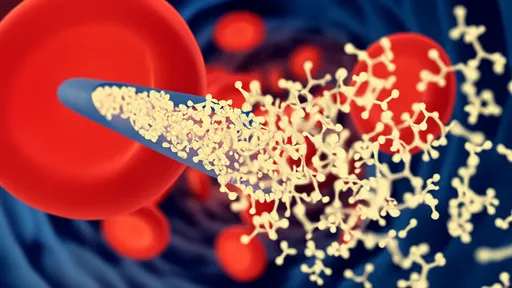
The technology centers around an injectable hydrogel that mimics the structure of certain marine organisms capable of capturing microscopic prey. When introduced into the bloodstream, this smart material forms a three-dimensional network that selectively ensnares CTCs while allowing normal blood cells to pass through unimpeded. Unlike conventional therapies that attack cancer cells directly, this method physically immobilizes them, effectively putting the brakes on metastasis before secondary tumors can form.
How the Biomimetic Trap Works
At the heart of the system lies a temperature-sensitive polymer that transitions from liquid to gel at body temperature. The material is infused with specialized ligands that recognize and bind to surface markers unique to CTCs. These binding sites were engineered based on studies of how barnacles adhere to surfaces in turbulent ocean environments—a process requiring both strength and specificity.
Once captured, the hydrogel's microenvironment triggers cellular changes that make the tumor cells more susceptible to immune detection. Early experiments show the trapped cells become "visible" to natural killer cells and macrophages, potentially enabling the body's own defenses to eliminate them without additional drugs.
From Concept to Clinical Potential
The research team spent years perfecting the hydrogel's composition, drawing inspiration from multiple biological systems. The final formulation combines elements of spider silk's tensile strength with the reversible adhesion properties of gecko feet. This unique combination allows the gel to maintain structural integrity under blood flow pressures while still releasing captured cells when subjected to specific medical interventions.
Animal trials have demonstrated remarkable efficacy. In mouse models of aggressive breast cancer, the hydrogel reduced metastatic lesions by 89% compared to control groups. Perhaps most impressively, the treatment showed effectiveness against multiple cancer types, suggesting the targeted surface markers are common across various malignancies.
Overcoming Traditional Limitations
Current approaches to preventing metastasis—such as chemotherapy or targeted radiation—often cause significant collateral damage to healthy tissues. The hydrogel system represents a paradigm shift by acting as a physical barrier rather than a toxic agent. Its localized effect could dramatically reduce the debilitating side effects associated with conventional cancer therapies.
Moreover, the material is designed to degrade safely within weeks, eliminating the need for surgical removal. As it breaks down, the hydrogel releases anti-inflammatory compounds that help repair any minor vessel damage caused during the implantation process.
The Road Ahead
While the results are promising, researchers caution that human trials are still several years away. The team is currently working to optimize the hydrogel's persistence in the bloodstream and refine its capture efficiency. Additional studies are examining whether combining the technology with immunotherapy could further enhance its cancer-fighting potential.
If successful, this bioinspired approach could fundamentally change how we treat advanced cancers. Rather than chasing metastatic cells throughout the body, clinicians might one day simply deploy this molecular "freeze gun" to stop them in their tracks—turning the tide in humanity's long battle against cancer's deadly spread.
The implications extend beyond oncology. The same principles could potentially be adapted to treat autoimmune diseases by capturing rogue immune cells, or to develop new diagnostic tools for early disease detection. As research progresses, this fusion of materials science and biological inspiration continues to open new frontiers in medical technology.
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 18, 2025
By /Aug 18, 2025
By /Aug 18, 2025